- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Roscovitine
别名: CYC202, Seliciclib, R-roscovitine
Roscovitine是一种有效的,选择性CDK抑制剂,作用于Cdc2,CDK2和CDK5时,无细胞试验中IC50分别为0.65 μM,0.7 μM和0.16 μM,对CDK4/6几乎没有作用。Phase 2。
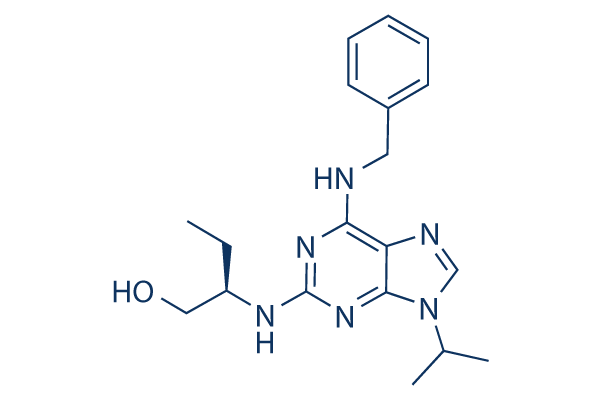
Roscovitine Chemical Structure
CAS: 186692-46-6


产品质控
批次:
纯度:
99.85%
99.85
Roscovitine相关产品
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| LP-1 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human LP-1 cells at 30 uM after 3 hrs using TUNEL staining by flow cytometry | 15958589 |
| LP-1 | Cytotoxicity assay | 20 to 30 uM | 24 hrs | Cytotoxicity against human LP-1 cells assessed as reduction of cell viability at 20 to 30 uM treated for 24 hrs followed by washout measured after total 72 hrs growth period alamar blue assay relative to control | 15958589 |
| LP-1 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human LP-1 cells assessed as reduction of RNA polymerase 2 phosphoserine 2 level at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| LP-1 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human LP-1 cells assessed as reduction of Mcl-1 protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| LP-1 | Apoptosis assay | 30 uM | 3 to 5 hrs | Induction of apoptosis in human LP-1 cells assessed as increase in level of cleaved PARP at 30 uM after 3 to 5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as increase in level of cleaved PARP at 30 uM after 5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as fast slow migrating hyperphosphorylated RNA polymerase 2O form at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| RPM18226 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human RPM18226 cells assessed as reduction of RNA polymerase 2 phosphoserine 2 level at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| RPM18226 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human RPM18226 cells assessed as reduction of Mcl-1 protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| RPM18226 | Apoptosis assay | 30 uM | 3 to 5 hrs | Induction of apoptosis in human RPM18226 cells assessed as increase in level of cleaved PARP at 30 uM after 3 to 5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human NCI-H929 cells assessed as changes in XIAP protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human NCI-H929 cells assessed as changes in survivin protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| RPM18226 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human RPM18226 cells at 30 uM after 3 hrs using TUNEL staining by flow cytometry | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as reduction of RNA polymerase 2 phosphoserine 2 level at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as dephosphorylation of pRb at S249/T252 at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Cytotoxicity assay | 20 to 30 uM | 16 hrs | Cytotoxicity against human NCI-H929 cells assessed as reduction of cell viability at 20 to 30 uM treated for 16 hrs followed by washout measured after total 72 hrs growth period alamar blue assay relative to control | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human NCI-H929 cells assessed as reduction of Mcl-1 protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human NCI-H929 cells assessed as changes in Bcl-2 protein level at 30 uM after 3 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 3 hrs | Induction of apoptosis in human NCI-H929 cells at 30 uM after 3 hrs using TUNEL staining by flow cytometry | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as reduction of RNA polymerase 2 phosphoserine 5 level at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as reduction of Hdm2 level at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| NCI-H929 | Apoptosis assay | 30 uM | 1.5 hrs | Induction of apoptosis in human NCI-H929 cells assessed as increase of p53 accumulation at 30 uM after 1.5 hrs by immunoblotting | 15958589 |
| HCT116 | Function assay | 30 to 40 umol/L | 24 hrs | Inhibition of cyclin A in human HCT116 cells assessed as decrease in protein level at 30 to 40 umol/L after 24 hrs by immunoblotting analysis | 21080703 |
| HCT116 | Function assay | 30 to 40 umol/L | 24 hrs | Inhibition of cyclin B in human HCT116 cells assessed as decrease in protein level at 30 to 40 umol/L after 24 hrs by immunoblotting analysis | 21080703 |
| HCT116 | Function assay | 30 to 40 umol/L | 24 hrs | Inhibition of cyclin D1 in human HCT116 cells assessed as decrease in protein level at 30 to 40 umol/L after 24 hrs by immunoblotting analysis | 21080703 |
| HCT116 | Function assay | 30 to 40 umol/L | 24 hrs | Inhibition of CDK2 in human HCT116 cells assessed as decrease in protein level at 30 to 40 umol/L after 24 hrs by immunoblotting analysis | 21080703 |
| HT-29 | Function assay | 2.5 to 40 uM | 24 hrs | Inhibition of retinoblastoma protein in human HT-29 cells assessed as reduction of cyclin A level at 2.5 to 40 uM after 24 hrs by immunoblotting | 21417417 |
| MCF7 | Cell cycle assay | 80 uM | 24 hrs | Cell cycle arrest in human MCF7 cells assessed as reduction of actively replicating DNA level at 80 uM after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 |
| MCF7 | Function assay | 20 uM | 24 hrs | Induction of p53-dependent transcriptional activity in human MCF7 cells assessed as increase of p21 WAF1 level at 20 uM after 24 hrs by immunofluorescence assay | 21417417 |
| RPMI8226 | Cell cycle assay | 80 uM | 24 hrs | Cell cycle arrest in human RPMI8226 cells assessed as reduction of actively replicating DNA level at 80 uM after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 |
| A549 | Apoptosis assay | 2 uM | 48 hrs | Induction of apoptosis in human A549 cells assessed as DNA fragmentation at 2 uM after 48 hrs by agarose gel electrophoresis | 23623491 |
| BJ | Function assay | 10 uM | 10 days | Suppression of senescence in human BJ cells assessed as increase in cell number at 10 uM after 10 days by senescence reversal assay | 24681986 |
| BJ | Function assay | 10 uM | 10 days | Inhibition of ataxia telangiectasia-mutated in human BJ cells assessed as increase in cell number at 10 uM after 10 days by senescence reversal assay | 24681986 |
| MCF7 | Function assay | 10 uM | 10 mins | Sensitization of infrared-induced DNA damage in human MCF7 cells assessed as reduction in colony formation at 10 uM pretreated for 10 mins followed by irradiation for 4 hrs measured after 10 days by crystal violet staining analysis | 26851505 |
| MCF7 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MCF7 cells assessed as accumulation at G2/M phase after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| RPMI8226 | Cell cycle assay | 24 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at G2/M phase after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| MCF7 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MCF7 cells assessed as decrease in S phase cell population after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| MCF7 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MCF7 cells assessed as accumulation at sub-G1 phase after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| RPMI8226 | Cell cycle assay | 24 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at sub-G1 phase after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| RPMI8226 | Cell cycle assay | 24 hrs | Cell cycle arrest in human RPMI8226 cells assessed as decrease in S phase cell population after 24 hrs using propidium iodide and BrdU staining by flow cytometry | 21417417 | |
| Sf9 | Function assay | 10 mins | Inhibition of His-6-tagged recombinant human CDK2/cyclinE expressed in baculovirus-infected sf9 cells using histone H1 as substrate after 10 mins by liquid scintillation counting in presence of [gamma-32P]ATP, IC50 = 0.1 μM. | 24417566 | |
| NCI-SNU-1 | Growth Inhibition Assay | IC50=31.1059 μM | SANGER | ||
| NKM-1 | Growth Inhibition Assay | IC50=31.1397 μM | SANGER | ||
| SIG-M5 | Growth Inhibition Assay | IC50=31.6833 μM | SANGER | ||
| SK-N-FI | Growth Inhibition Assay | IC50=31.7535 μM | SANGER | ||
| LOUCY | Growth Inhibition Assay | IC50=32.1253 μM | SANGER | ||
| Calu-6 | Growth Inhibition Assay | IC50=32.4745 μM | SANGER | ||
| GOTO | Growth Inhibition Assay | IC50=32.9129 μM | SANGER | ||
| NCI-H526 | Growth Inhibition Assay | IC50=33.4936 μM | SANGER | ||
| RKO | Growth Inhibition Assay | IC50=33.5969 μM | SANGER | ||
| NCI-H64 | Growth Inhibition Assay | IC50=33.8597 μM | SANGER | ||
| LP-1 | Growth Inhibition Assay | IC50=33.8908 μM | SANGER | ||
| KGN | Growth Inhibition Assay | IC50=34.2524 μM | SANGER | ||
| NCI-H2141 | Growth Inhibition Assay | IC50=34.6533 μM | SANGER | ||
| TE-10 | Growth Inhibition Assay | IC50=34.9422 μM | SANGER | ||
| K5 | Growth Inhibition Assay | IC50=35.0861 μM | SANGER | ||
| IMR-5 | Growth Inhibition Assay | IC50=35.3139 μM | SANGER | ||
| TE-441-T | Growth Inhibition Assay | IC50=36.1148 μM | SANGER | ||
| TE-6 | Growth Inhibition Assay | IC50=36.3246 μM | SANGER | ||
| MOLT-4 | Growth Inhibition Assay | IC50=36.3276 μM | SANGER | ||
| COLO-684 | Growth Inhibition Assay | IC50=37.012 μM | SANGER | ||
| LU-139 | Growth Inhibition Assay | IC50=37.1856 μM | SANGER | ||
| OPM-2 | Growth Inhibition Assay | IC50=37.2949 μM | SANGER | ||
| ML-2 | Growth Inhibition Assay | IC50=37.6712 μM | SANGER | ||
| RS4-11 | Growth Inhibition Assay | IC50=37.7069 μM | SANGER | ||
| MONO-MAC-6 | Growth Inhibition Assay | IC50=38.2477 μM | SANGER | ||
| NCI-H345 | Growth Inhibition Assay | IC50=38.9106 μM | SANGER | ||
| NTERA-S-cl-D1 | Growth Inhibition Assay | IC50=39.5842 μM | SANGER | ||
| NCI-H1882 | Growth Inhibition Assay | IC50=40.5998 μM | SANGER | ||
| LC-1F | Growth Inhibition Assay | IC50=41.5705 μM | SANGER | ||
| HT | Growth Inhibition Assay | IC50=42.0028 μM | SANGER | ||
| MLMA | Growth Inhibition Assay | IC50=42.2787 μM | SANGER | ||
| DG-75 | Growth Inhibition Assay | IC50=42.6546 μM | SANGER | ||
| GI-ME-N | Growth Inhibition Assay | IC50=42.6671 μM | SANGER | ||
| MS-1 | Growth Inhibition Assay | IC50=42.893 μM | SANGER | ||
| CGTH-W-1 | Growth Inhibition Assay | IC50=44.9697 μM | SANGER | ||
| NCI-H209 | Growth Inhibition Assay | IC50=46.0115 μM | SANGER | ||
| LB2518-MEL | Growth Inhibition Assay | IC50=47.0448 μM | SANGER | ||
| DU-4475 | Growth Inhibition Assay | IC50=48.4937 μM | SANGER | ||
| LB2241-RCC | Growth Inhibition Assay | IC50=48.6202 μM | SANGER | ||
| LB771-HNC | Growth Inhibition Assay | IC50=48.9212 μM | SANGER | ||
| NCI-H82 | Growth Inhibition Assay | IC50=31.0135 μM | SANGER | ||
| NCI-H510A | Growth Inhibition Assay | IC50=30.0329 μM | SANGER | ||
| ES3 | Growth Inhibition Assay | IC50=29.9582 μM | SANGER | ||
| BB30-HNC | Growth Inhibition Assay | IC50=29.9483 μM | SANGER | ||
| KM12 | Growth Inhibition Assay | IC50=29.6239 μM | SANGER | ||
| GI-1 | Growth Inhibition Assay | IC50=29.0113 μM | SANGER | ||
| NOS-1 | Growth Inhibition Assay | IC50=28.9733 μM | SANGER | ||
| TE-8 | Growth Inhibition Assay | IC50=28.908 μM | SANGER | ||
| TE-9 | Growth Inhibition Assay | IC50=28.7969 μM | SANGER | ||
| HL-60 | Growth Inhibition Assay | IC50=27.9869 μM | SANGER | ||
| QIMR-WIL | Growth Inhibition Assay | IC50=27.9144 μM | SANGER | ||
| KARPAS-299 | Growth Inhibition Assay | IC50=26.8646 μM | SANGER | ||
| KURAMOCHI | Growth Inhibition Assay | IC50=26.8082 μM | SANGER | ||
| BL-41 | Growth Inhibition Assay | IC50=25.9597 μM | SANGER | ||
| NCI-H2126 | Growth Inhibition Assay | IC50=25.6529 μM | SANGER | ||
| HOP-62 | Growth Inhibition Assay | IC50=25.4425 μM | SANGER | ||
| IST-SL2 | Growth Inhibition Assay | IC50=24.5343 μM | SANGER | ||
| HH | Growth Inhibition Assay | IC50=24.3819 μM | SANGER | ||
| LS-513 | Growth Inhibition Assay | IC50=23.5179 μM | SANGER | ||
| EB-3 | Growth Inhibition Assay | IC50=23.1831 μM | SANGER | ||
| ACN | Growth Inhibition Assay | IC50=21.3389 μM | SANGER | ||
| NOMO-1 | Growth Inhibition Assay | IC50=21.2008 μM | SANGER | ||
| ES8 | Growth Inhibition Assay | IC50=21.06 μM | SANGER | ||
| CESS | Growth Inhibition Assay | IC50=20.8549 μM | SANGER | ||
| BL-70 | Growth Inhibition Assay | IC50=20.3274 μM | SANGER | ||
| MHH-PREB-1 | Growth Inhibition Assay | IC50=20.0356 μM | SANGER | ||
| BC-1 | Growth Inhibition Assay | IC50=19.1198 μM | SANGER | ||
| LC4-1 | Growth Inhibition Assay | IC50=18.8734 μM | SANGER | ||
| COLO-320-HSR | Growth Inhibition Assay | IC50=18.7688 μM | SANGER | ||
| A101D | Growth Inhibition Assay | IC50=18.3208 μM | SANGER | ||
| BC-3 | Growth Inhibition Assay | IC50=18.0305 μM | SANGER | ||
| TGW | Growth Inhibition Assay | IC50=17.8124 μM | SANGER | ||
| JAR | Growth Inhibition Assay | IC50=17.0152 μM | SANGER | ||
| HD-MY-Z | Growth Inhibition Assay | IC50=16.8246 μM | SANGER | ||
| NCI-H1304 | Growth Inhibition Assay | IC50=16.3601 μM | SANGER | ||
| OS-RC-2 | Growth Inhibition Assay | IC50=15.8382 μM | SANGER | ||
| OCI-AML2 | Growth Inhibition Assay | IC50=15.6482 μM | SANGER | ||
| HCC1599 | Growth Inhibition Assay | IC50=14.5975 μM | SANGER | ||
| SCC-3 | Growth Inhibition Assay | IC50=14.2956 μM | SANGER | ||
| RPMI-6666 | Growth Inhibition Assay | IC50=13.9121 μM | SANGER | ||
| MEG-01 | Growth Inhibition Assay | IC50=13.8379 μM | SANGER | ||
| Raji | Growth Inhibition Assay | IC50=13.7894 μM | SANGER | ||
| RPMI-8402 | Growth Inhibition Assay | IC50=13.6262 μM | SANGER | ||
| GCIY | Growth Inhibition Assay | IC50=12.8613 μM | SANGER | ||
| 697 | Growth Inhibition Assay | IC50=12.6007 μM | SANGER | ||
| D-247MG | Growth Inhibition Assay | IC50=12.3516 μM | SANGER | ||
| NB1 | Growth Inhibition Assay | IC50=12.3308 μM | SANGER | ||
| COR-L279 | Growth Inhibition Assay | IC50=12.2907 μM | SANGER | ||
| LB831-BLC | Growth Inhibition Assay | IC50=11.5624 μM | SANGER | ||
| ST486 | Growth Inhibition Assay | IC50=10.351 μM | SANGER | ||
| SK-UT-1 | Growth Inhibition Assay | IC50=10.35 μM | SANGER | ||
| BB65-RCC | Growth Inhibition Assay | IC50=9.97495 μM | SANGER | ||
| KARPAS-422 | Growth Inhibition Assay | IC50=9.96336 μM | SANGER | ||
| Becker | Growth Inhibition Assay | IC50=9.46082 μM | SANGER | ||
| KS-1 | Growth Inhibition Assay | IC50=9.45785 μM | SANGER | ||
| JiyoyeP-2003 | Growth Inhibition Assay | IC50=8.50264 μM | SANGER | ||
| NCCIT | Growth Inhibition Assay | IC50=7.55482 μM | SANGER | ||
| MRK-nu-1 | Growth Inhibition Assay | IC50=7.12969 μM | SANGER | ||
| A3-KAW | Growth Inhibition Assay | IC50=5.76116 μM | SANGER | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 15958589 | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 21080703 | ||
| Caco2 | Cell cycle assay | Cell cycle arrest in human Caco2 cells assessed as accumulation at G1/S phase by Hoechst staining based fluorescence assay | 28214231 | ||
| HaCaT | Cell cycle assay | Cell cycle arrest in human HaCaT cells assessed as accumulation at G1/S phase by Hoechst staining based fluorescence assay | 28214231 | ||
| HuH7 | Cell cycle assay | Cell cycle arrest in human HuH7 cells assessed as accumulation at G1/S phase by Hoechst staining based fluorescence assay | 28214231 | ||
| PC3 | Cell cycle assay | Cell cycle arrest in human PC3 cells assessed as accumulation at G2/M phase by Hoechst staining based fluorescence assay | 28214231 | ||
| MDA-MB-231 | Cell cycle assay | Cell cycle arrest in human MDA-MB-231 cells assessed as accumulation at G1/S phase by Hoechst staining based fluorescence assay | 28214231 | ||
| HCT116 | Cell cycle assay | Cell cycle arrest in human HCT116 cells assessed as accumulation at G1/S phase by Hoechst staining based fluorescence assay | 28214231 | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 28557430 | ||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | ||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | ||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | ||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | ||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | ||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 30199702 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Roscovitine是一种有效的,选择性CDK抑制剂,作用于Cdc2,CDK2和CDK5时,无细胞试验中IC50分别为0.65 μM,0.7 μM和0.16 μM,对CDK4/6几乎没有作用。Phase 2。 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | Roscovitine作用于细胞周期蛋白依赖性激酶具有高效性和高度选择性,作用于cdc2/cyclin B, cdk2/cyclin A, cdk2/cyclin E和cdk5/p53时IC50分别为0.65,0.7,0.7和0.16 μM。纳摩尔级Roscovitine作用于海星卵母细胞和海胆胚胎,可逆抑制在前中期间转变, 在体外作用于非洲爪蟾卵提取物,抑制M期促进因子活性和体外DNA合成,且抑制哺乳动物细胞系增殖,IC50为16 μM。[1] 浓度为7.5, 12.5和 25 mM的 Roscovitine作用于肾小球系膜细胞,导致CDK2活性分别降低25,50% 和100%,这种作用存在剂量依赖性。[2] 最新研究显示Roscovitine作用于盘基网柄菌,抑制cdk5激酶活性,细胞增殖,多细胞发展,和cdk5核转运, 不会影响cdk5蛋白表达。[3] |
|||
|---|---|---|---|---|
| 激酶实验 | 酶实验 | |||
| 激酶活性实验在30oC下buffer C中进行。从数据中除去空白值,在10分钟的温育期中测定渗透到蛋白受体中的磷酸摩尔数,来计算活性。对照组用适当稀释的DMSO处理。在一些情况下, SDS/PAGE后通过自动射线照相术测定底物磷酸化。p34cdc2/cyclin B通过亲和色谱从 M期海星卵母细胞中纯化。使用 1 mg 组蛋白Hl/mL,在15 μM [γ-32P]ATP存在时进行实验,终浓度为 30 μL。在 30oC下温育10分钟, 25-μL上清液 转移到Whatman P81磷酸纤维素纸上, 20秒后, 用10mL磷酸/L水冲洗过滤器5次,每次至少5分钟。湿式过滤器转移到 6 mL闪烁管,加入5 mL ACS闪烁液,使用Packard 计数器测定放射性。测定在10分钟温育期中组蛋白H1渗透放入磷酸摩尔数评估激酶活性或者最大活性百分数。感染不同杆状病毒的sf9昆虫细胞抽提物中再生p33cdk2/cyclin A和p33cdk2/cyclinE。Cyclins A 和E是谷胱甘肽S-转移酶融合蛋白,复合体从谷胱甘肽-琼脂糖珠上纯化。使用 1 mg/mL 组蛋白Hl/mL,在15 μM [γ-32P]ATP存在时,进行激酶活性实验10分钟,终体积为30 μL,测定p34cdc2/cyclin B激酶。p33cdk5/p35从牛脑中纯化,除了Mono S-色层分离一步法。 Superose 12柱的活性片段汇集,终浓度为25 μg 酶/mL。使用1 mg/mL 组蛋白Hl, 在15 μM [γ-32P]ATP存在时,进行激酶活性实验10分钟,终体积为 30 μL,测定p34cdc2/cyclin B激酶。 | ||||
| 细胞实验 | 细胞系 | 白血病, 非小细胞肺癌,结肠癌, 中枢神经系统肿瘤, 恶性黑色素瘤,卵巢癌,肾癌, 前列腺癌,胸腺癌细胞系 | ||
| 浓度 | 0.01到100 μM | |||
| 孵育时间 | 48小时 | |||
| 方法 | 包括9种肿瘤类型的60种人类肿瘤细胞系培养24小时,然后用 0.01-100 μM Roscovitine持续处理48小时。进行sulforhodaminine B蛋白实验测评毒性。 |
|||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | p-Rb / p-CDK2 / CDK2 / Cyclin D1 / Cyclin A2 / ERα / ERβ/ AIB1 / PELP1 pT231-tau / pS202-tau / tau |
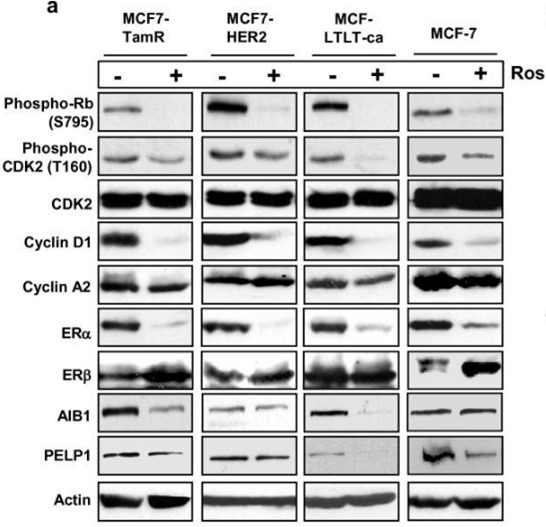
|
21834972 | |
| Immunofluorescence | E2F1 / FASN / Bmi1 / Cyclin D2 / CDK2 / CDK4 CDK1 / Smek2 / FUBP1 / Cdc20 |
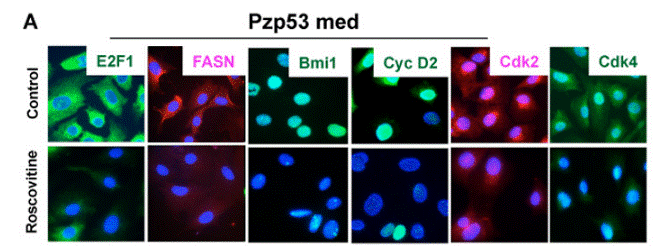
|
20890301 | |
| Growth inhibition assay | Cell viability |
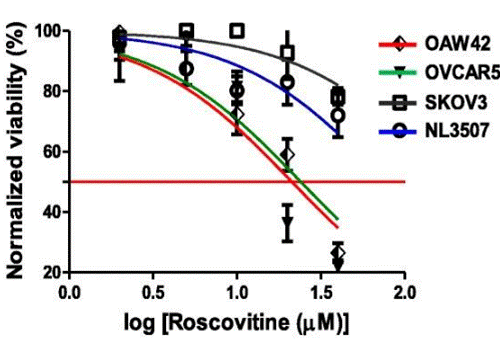
|
29996940 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | Roscovitine按50 mg/kg剂量作用于Ewing's肉瘤家族(ESFT)移植瘤,明显抑制肿瘤生长。[4] Roscovitine作用于携带MCF7移植瘤的裸鼠,增强抗癌Doxorubicin抗癌效果,不会提高毒性,机制是使细胞周期停滞而不是引起凋亡。[5] |
|
|---|---|---|
| 动物实验 | Animal Models | 右后侧皮下注射A4573细胞的CD1 nu/nu鼠 |
| Dosages | ≤50 mg/kg | |
| Administration | 腹腔注射 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02649751 | Terminated | Cystic Fibrosis |
University Hospital Brest|ManRos Therapeutics|Cyclacel Pharmaceuticals Inc. |
February 22 2016 | Phase 2 |
|
化学信息&溶解度
| 分子量 | 354.45 | 分子式 | C19H26N6O |
| CAS号 | 186692-46-6 | SDF | Download Roscovitine SDF |
| Smiles | CCC(CO)NC1=NC(=C2C(=N1)N(C=N2)C(C)C)NCC3=CC=CC=C3 | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 71 mg/mL ( (200.31 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 71 mg/mL (200.31 mM) Water : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项
常见问题及建议解决方法
问题 1:
How can I reconstitute the drug for in vivo studies?
回答:
S1153 in 1% DMSO+10% Tween 80+20% N-N-dimethylacetamide+PEG 400 is a clear solution which is okay for injection. And S1153 in 1% DMSO+30% polyethylene glycol+1% Tween 80 at 30mg/ml is a suspension, which is fine for oral gavage.