- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
GSK1349572 (Dolutegravir)
别名: GSK1349572,S/GSK1349572 中文名称:度鲁特韦,德罗格韦
Dolutegravir是一种两个金属结合的HIV integrase抑制剂,无细胞试验中IC50为2.7 nM,对耐Raltegravir的显著突变体Y143R,Q148K,N155H,和G140S/Q148H具有适度的活性。

GSK1349572 (Dolutegravir) Chemical Structure
CAS: 1051375-16-6


产品质控
批次:
纯度:
99.85%
99.85
常与GSK1349572 (Dolutegravir)一起在实验中被使用的化合物
GSK1349572 (Dolutegravir)相关产品
| 相关靶点 | HIV-1 Integrase HIV-2 Integrase HIV Integrase | 点击展开 |
|---|---|---|
| 相关产品 | MK-2048 BMS-707035 | 点击展开 |
| 相关化合物库 | 抗感染化合物库 抗生素化合物库 抗病毒化合物库 抗寄生虫药物库 肠道微生物代谢物库 | 点击展开 |
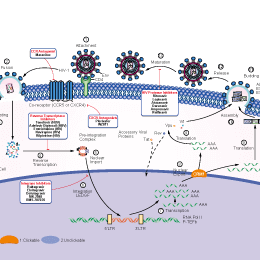
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| HOS | Antiviral assay | 3 hrs | Antiviral activity against HIV-1 harboring wild type integrase infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.0016 μM. | 24901667 | |
| HEK293T | Antiviral assay | 2 days | Antiviral activity against pseudo Human immunodeficiency virus infected in HEK293T cells after 2 days, IC50 = 0.0017 μM. | 23845180 | |
| MT4 | Antiviral assay | 4 to 5 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT4 cells after 4 to 5 days by bioluminescence assay, IC50 = 0.002 μM. | 23845180 | |
| HOS | Antiviral assay | 3 hrs | Antiviral activity against raltegravir-resistant HIV-1 harboring integrase N155H mutant infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.0036 μM. | 24901667 | |
| HOS | Antiviral assay | 3 hrs | Antiviral activity against raltegravir-resistant HIV-1 harboring integrase Y143R mutant infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.0043 μM. | 24901667 | |
| HOS | Antiviral assay | 3 hrs | Antiviral activity against raltegravir-resistant HIV-1 harboring integrase G140S/Q148H double mutant infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.0058 μM. | 24901667 | |
| HOS | Antiviral assay | 3 hrs | Antiviral activity against INSTI-resistant HIV-1 harboring integrase R263K mutant infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.011 μM. | 24901667 | |
| HOS | Antiviral assay | 3 hrs | Antiviral activity against INSTI-resistant HIV-1 harboring integrase G118R mutant infected in human HOS cells pretreated with compound for 3 hrs by single-round HIV-1 infectivity assay, EC50 = 0.013 μM. | 24901667 | |
| P4R5 MAGI | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in CD4/CXCR4/CCR5 expressing human P4R5 MAGI cells preincubated with cells for 24 hrs followed by viral infection measured after 48 hrs by beta-galactosidase reporter gene assay, EC50 = 0.02 μM. | 30031976 | |
| P4R5 | Antiviral assay | 24 hrs | Antiviral activity against HIV1 infected in CD4/CXCR4/CCR5 expressing human P4R5 cells assessed as inhibition of viral replication preincubated with cells for 24 hrs followed by viral infection measured after 48 hrs by beta-galactosidase reporter gene ass, EC50 = 0.02 μM. | 28525279 | |
| HEK293T | Antiviral assay | 2 days | Antiviral activity against pseudo Human immunodeficiency virus infected in HEK293T cells after 2 days in presence of human serum albumin, IC50 = 0.022 μM. | 23845180 | |
| Vero E6 | Antiviral assay | 2 days | Antiviral efficacy against SARS-CoV-2 (strain BavPat1) in Vero E6 cells assessed by inhibition of viral RNA replication measured by RT-PCR after 2 days, EC50 = 22.04 μM. | ChEMBL | |
| Vero E6 | Antiviral assay | 2 days | Antiviral efficacy against SARS-CoV-2 (strain BavPat1) in Vero E6 cells assessed by inhibition of viral RNA replication measured by RT-PCR after 2 days, EC90 = 42.81 μM. | ChEMBL | |
| MDCK2 cells | Function assay | Inhibition of human OCT2 expressed in MDCK2 cells using [14C]metformin as substrate by liquid scintillation counting analysis | 23132334 | ||
| MDCK2 | Function assay | Inhibition of human OCT2 expressed in MDCK2 cells using [14C]metformin as substrate by liquid scintillation counting analysis, IC50 = 1.9 μM. | 23132334 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Dolutegravir是一种两个金属结合的HIV integrase抑制剂,无细胞试验中IC50为2.7 nM,对耐Raltegravir的显著突变体Y143R,Q148K,N155H,和G140S/Q148H具有适度的活性。 | ||
|---|---|---|---|
| 特性 | 新一代两种金属结合的HIV整合酶链转移抑制剂。 | ||
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | S/GSK1349572对来自整合酶抑制剂-天然HIV-2感染患者的9种临床分离株表现出有效的抑制作用,EC50范围为0.2 nM -1.4 nM。 在体外,S/GSK1349572抑制重组HIV-1整合酶催化的链转移,IC50为2.7 nM。此外,S/GSK1349572有效抑制细胞中HIV复制,如作用于感染自身灭活的PHIV慢病毒载体的外周血单核细胞(PBMCs),MT-4 细胞和CIP4细胞,EC50分别为0.51 nM,0.71 nM和2.2 nM。 在体外,S/GSK1349572对5种不同的非核苷逆转录抑制剂抗性病毒,或核苷逆转录抑制剂抗性病毒表现出有效的活性,EC50范围为1.3 nM -2.1 nM。与抗野生型病毒类似,S/GSK1349572对两种蛋白酶抑制剂抗性病毒表现出同样的活性,EC50为0.36 nM和0.37 nM。 |
|||
|---|---|---|---|---|
| 激酶实验 | 体外链转移试验 | |||
| S/GSK1349572和其他INIs的抑制效能通过链转移试验使用重组HIV整合酶测量。整合酶和生物素化预处理的供体DNA-链霉亲和素包被的闪烁迫近测定(SPA)珠复合物,通过将2 μM纯化的重组整合酶与0.66 μM生物素化供体DNA-4 mg/mL链霉亲和素包被的SPA珠在25 mM 吗啡啉丙磺酸钠(MOPS) (pH 7.2),23 mM NaCl,和10 mM MgCl2于37 °C下培养5分钟制备。将这些珠子离心,并与稀释的INIs在37 °C下预培养60分钟。然后加入3H-标记的靶DNA底物,得到底物终浓度为7 nM,将链转移反应混合物在37 °C下培育25到45分钟,使供体DNA到放射性标记的靶DNA链转移线性增加。信号使用Wallac MicroBeta闪烁酶标仪读取。 | ||||
| 细胞实验 | 细胞系 | MT-4 | ||
| 浓度 | 0到10 μM | |||
| 孵育时间 | 4天或5天 | |||
| 方法 | 以500000 或 600000 /mL的密度指数生长的MT-4细胞用HIV-1菌株IIIB感染,病毒感染率为0.001,或50%组织培养感染剂量为4-10。随后将细胞等份接种到含有不同浓度S/GSK1349572的96孔板。培养4到5天后,抗病毒活性通过细胞活性试验测定,即用CellTiter-Glo荧光试剂测量生物发光,或使用黄色四唑MTT试剂[3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐]测量560和 690 nm下的吸光度确定。 |
|||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Dose-response infectivity curves | NL4.3IN(WT) / NL4.3IN(S230R) virus |

|
29617824 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06281834 | Not yet recruiting | Pediatric HIV Infection|Latent Tuberculosis |
Brigham and Women''s Hospital|APIN Public Health Initiatives|University of Cape Town |
May 2024 | Phase 1 |
| NCT05122026 | Recruiting | HIV Seropositivity|Pregnancy|Tuberculosis Infection |
The Aurum Institute NPC|Johns Hopkins University|Weill Medical College of Cornell University|University of Washington |
January 17 2024 | Phase 1|Phase 2 |
| NCT05069688 | Recruiting | Pediatric HIV Infection|Tuberculosis Infection |
Brigham and Women''s Hospital|APIN Public Health Initiatives|University of Cape Town |
July 7 2023 | Phase 1 |
| NCT05122767 | Recruiting | Tuberculosis|HIV |
The Aurum Institute NPC|Johns Hopkins University |
May 24 2023 | Phase 1|Phase 2 |
|
化学信息&溶解度
| 分子量 | 419.38 | 分子式 | C20H19F2N3O5 |
| CAS号 | 1051375-16-6 | SDF | Download GSK1349572 (Dolutegravir) SDF |
| Smiles | CC1CCOC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 84 mg/mL ( (200.29 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Water : Insoluble Ethanol : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项