- 抑制剂
- 化合物库
- 热卖化合物库
- 定制您的化合物库
- 临床和上市相关
- 活性化合物总库
- 抑制剂相关
- 天然产物和药食同源相关
- 代谢相关
- 细胞死亡相关
- 按信号通路分类
- 按疾病分类
- 抗感染和抗病毒相关
- 神经和免疫相关
- 片段和共价相关
- FDA药物库
- 临床I期后及FDA药物库
- 临床前和临床药物库
- 已知活性药物库-I
- 生物活性库Ⅱ
- 激酶抑制剂库
- 多样性化合物母核库
- 天然产物库
- 人内源代谢化合物库
- 生物碱类化合物库New
- 血管生成相关化合物库
- 抗衰老化合物库
- 抗阿尔茨海默病化合物库
- 抗生素化合物库
- 抗肿瘤化合物库
- 抗癌化合物库-Ⅱ
- 抗癌代谢化合物库
- 抗心血管疾病化合物库
- 抗糖尿病化合物库
- 抗感染化合物库
- 抗氧化化合物库
- 抗寄生虫药物库
- 抗病毒化合物库
- 凋亡分子化合物库
- 自噬化合物库
- 钙通道阻滞剂库New
- Cambridge抗癌化合物库
- 糖代谢化合物库New
- 细胞周期化合物库
- 血脑屏障通透化合物库
- 共价抑制剂库
- 细胞因子抑制剂库New
- 细胞骨架信号通路化合物库
- DNA损伤/ DNA修复化合物库
- 类药性化合物库
- 内质网应激库
- 表观遗传化合物库
- 外泌体分泌相关化合物库New
- FDA抗癌药物库New
- 铁死亡化合物库
- 黄酮类化合物库
- 片段库
- 谷氨酰胺代谢化合物库
- 糖酵解化合物库
- GPCR小分子化合物库
- 肠道微生物代谢物库
- HIF-1信号通路化合物库
- 高选择性抑制剂库
- 组蛋白修饰化合物库
- 新药发现高通量筛选库
- 人类激素相关化合物库New
- 人转录因子化合物库New
- 免疫/炎症分子化合物库
- 抑制剂库
- 离子通道配体库
- JAK-STAT信号通路库
- 脂代谢化合物库New
- 大环化合物库
- MAPK抑制剂库
- 药食同源化合物库
- 代谢化合物库
- 甲基化化合物库
- 小鼠代谢化合物库New
- 天然有机化合物库
- 神经信号化合物库
- NF-κB信号通路库
- 核苷类似物库
- 肥胖化合物库
- 氧化应激化合物库New
- 植物提取物库
- 表型筛选库
- PI3K/Akt 抑制剂库
- 蛋白酶抑制剂库
- 蛋白-蛋白互作(PPI)抑制剂库
- 细胞焦亡化合物库
- 小分子免疫肿瘤化合物库
- 线粒体靶向化合物库New
- 干细胞分化化合物库New
- 干细胞小分子化合物库
- 天然酚类化合物库New
- 天然萜类化合物库New
- TGF-beta/Smad信号通路库
- 中药化合物库
- 酪氨酸激酶抑制剂分子库
- 泛素化化合物库
- 定制化合物库-1
- 定制化合物库-2
- 定制化合物库-3
- 定制化合物库-4
- 定制化合物库-5
- 定制化合物库-6
-
定制您的化合物库
通过在我们的库存中挑选化合物来建立合适的化合物库,用于您的研究工作。
请通过info@selleck.cn与我们联系,定制你所需要的化合物库。
您可以选择:
- 抗体
- 生物试剂
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- 蛋白实验
- Protein A/G免疫沉淀磁珠
- Anti-DYKDDDDK Tag免疫磁珠
- Anti-DYKDDDDK Tag亲和凝胶
- Anti-Myc免疫磁珠
- Anti-HA免疫磁珠
- 磁力架
- Poly DYKDDDDK Tag多肽
- 细胞核与细胞浆蛋白抽提试剂盒
- 免疫磁珠
- 蛋白酶抑制剂Cocktail
- 蛋白酶抑制剂Cocktail(DMSO储液)
- 磷酸酶抑制剂Cocktail
- 新产品
- 联系我们
Ceralasertib (AZD6738)
Ceralasertib (AZD6738) 是一种口服具有活性的,选择性 ATR 激酶抑制剂,IC50 为 1 nM。Phase 1/2。
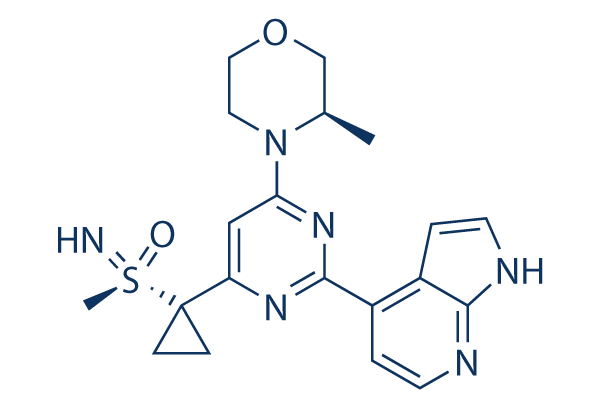
Ceralasertib (AZD6738) Chemical Structure
CAS: 1352226-88-0


产品质控
批次:
纯度:
99.71%
99.71
常与Ceralasertib (AZD6738)一起在实验中被使用的化合物
Ceralasertib (AZD6738)相关产品
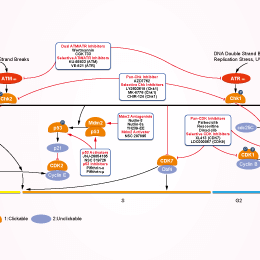
相关信号通路图
细胞实验数据示例
| 细胞系 | 实验类型 | 给药浓度 | 孵育时间 | 活性描述 | 文献信息(PMID) |
|---|---|---|---|---|---|
| LoVo cells | Function assay | 75 mg/kg | 8 hrs | Cp = 2.6 μM | 30346772 |
| HT-29 cells | Cytotoxicity assay | 72 hrs | GI50 = 2.6 μM | 30346772 | |
| LoVo cells | Function assay | 25 mg/kg | 8 hrs | Cp = 0.74 μM | 30346772 |
| LoVo cells | Function assay | 50 mg/kg | 8 hrs | Cp = 2.2 μM | 30346772 |
| LoVo cells | Cytotoxicity assay | 72 hrs | GI50 = 0.44 μM | 30346772 | |
| LICR-LON-HN4 and LICR-LON-HN5 cells | Function assay | 0.03, 0.1, 0.3, 1, 3, 10 μM | AZD6738 inhibition of ATR through loss of downstream phosphorylation of CHK1 on Ser345. | 30057890 | |
| K8484 cells | Function assay | 2 μM | 7 hours | In K8484 cells, AZD6738 at 2 µM completely prevented LY-188011-induced Chk1 phosphorylation on Serine 345, the downstream ATR target. | 29891488 |
| SNU-601 cells | Cell growth inhibition assay | 0-1 μmol/L | 5 days | The S and sub-G1 populations of SNU-601 cells were dramatically and dose-dependently increased by AZD6738. | 28138034 |
| HT29 cells | Function assay | 60 mins | IC50 = 0.074 μM | 30346772 | |
| LoVo cells | Function assay | 24 h | Reduction in cell count; a proportion of the cell population are (in addition to cell cycle arrest) undergoing apoptosis when exposed to drug at concentrations greater than 3 μM | 26310312 | |
| breast cancer cell lines | Cell growth inhibition assay | 0.125, 0.25, 0.5 and 1.0 μM | 5 days | IC50 values ranged from 0.3 to >1 μmol/L | 27501113 |
| MDA-MB-468 cells | Function assay | IC50 = 5.7 μM | 30346772 | ||
| 点击查看更多细胞系数据 | |||||
生物活性
| 产品描述 | Ceralasertib (AZD6738) 是一种口服具有活性的,选择性 ATR 激酶抑制剂,IC50 为 1 nM。Phase 1/2。 | ||
|---|---|---|---|
| 靶点 |
|
| 体外研究(In Vitro) | ||||
| 体外研究活性 | 在四个Kras突变细胞系:H23,H460,A549,和H358中,AZD6738抑制ATR激酶活性,并损害细胞活性。在ATM缺失的H23细胞中,AZD6738强烈增强顺铂诱导快速细胞死亡的作用。在p53 或 ATM缺失的细胞中,AZD6738治疗引起复制叉停滞和未修复DNA损伤的积累,导致有丝分裂障碍,从而使细胞死亡。 |
|||
|---|---|---|---|---|
| 细胞实验 | 细胞系 | H23,H460,A549,和 H358 细胞 | ||
| 浓度 | ~30 μM | |||
| 孵育时间 | 48小时 | |||
| 方法 | 细胞在白色壁,透明底的96孔板中与指示剂量的AZD6738,NSC 119875,LY188011,或它们的组合处理48小时。ATP水平通过CellTiter-Glo发光细胞活性试验和Safire2酶标仪测量代替品活性评估。进一步分析之前,原始数据对本底发光进行校正。对于AZD6738治疗,在GraphPad Prism 6中将对数转化(x=log(x))的数据归一化为未处理对照组的平均值,通过非线性回归(log(抑制剂) vs. 对可变斜率的响应值) 生成对数剂量响应曲线。GI50值,定义为Y = 50%时,X的剂量,根据剂量反应曲线推导得出。 |
|||
| 实验图片 | 检测方法 | 检测指标 | 实验图片 | PMID |
| Western blot | pCHK1 / pCDC25c / pRPA32 / γH2AX / pHH3 / cleaved caspase-3 / RAD51 ATM pSer1981 / ATM / ATR / Chk1 pSer345 / Chk1 / Chk2 pThr68 / Chk2 |
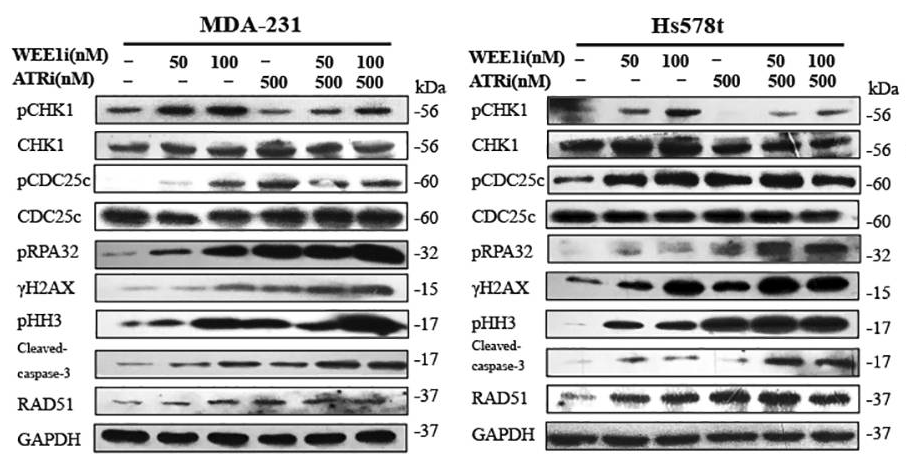
|
29605721 | |
| Immunofluorescence | γH2AX / RAD51 53BP1 |
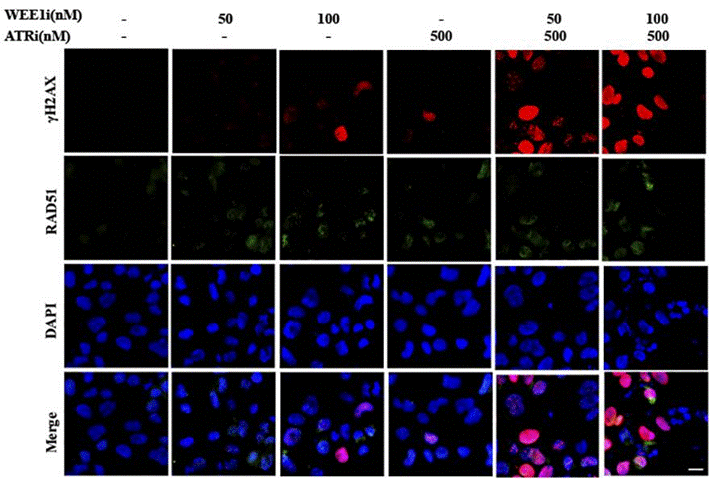
|
29605721 | |
| Growth inhibition assay | Cell viability IC50 |
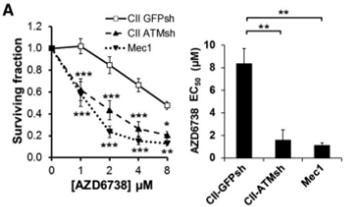
|
26563132 | |
| 体内研究(In Vivo) | ||
| 体内研究活性 | 在负荷H460和H23肿瘤的裸鼠中,AZD6738 (50 mg/kg, p.o.)导致肿瘤生长抑制(TGI),结合顺铂引起ATM缺失的H23肿瘤快速退化。在负荷LoVo异种移植物的裸鼠中,AZD6738 (50 mg/kg) + IR (2 Gy)的组合避免了毒性,同时仍保持疗效。 |
|
|---|---|---|
| 动物实验 | Animal Models | 负荷 H23 或 H460 异种移植物的雌性无胸腺裸鼠 |
| Dosages | 25 或 50 mg/kg | |
| Administration | p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05941897 | Active not recruiting | Advanced or Metastatic NSCLC |
AstraZeneca |
June 21 2023 | Phase 2 |
| NCT05514132 | Active not recruiting | Advanced Solid Tumours |
AstraZeneca |
September 23 2022 | Phase 1 |
| NCT05450692 | Recruiting | Advanced or Metastatic Non-Small Cell Lung Cancer |
AstraZeneca|Parexel |
September 15 2022 | Phase 3 |
| NCT05061134 | Active not recruiting | Melanoma |
AstraZeneca |
August 11 2022 | Phase 2 |
| NCT05469919 | Active not recruiting | Advanced Solid Malignancies |
AstraZeneca |
June 9 2022 | Phase 1 |
| NCT04704661 | Recruiting | Advanced Breast Carcinoma|Advanced Colon Carcinoma|Advanced Colorectal Carcinoma|Advanced Endometrial Carcinoma|Advanced Gastric Carcinoma|Advanced Gastroesophageal Junction Adenocarcinoma|Advanced Malignant Solid Neoplasm|Advanced Salivary Gland Carcinoma|Anatomic Stage III Breast Cancer AJCC v8|Anatomic Stage IIIA Breast Cancer AJCC v8|Anatomic Stage IIIB Breast Cancer AJCC v8|Anatomic Stage IIIC Breast Cancer AJCC v8|Anatomic Stage IV Breast Cancer AJCC v8|Clinical Stage III Gastric Cancer AJCC v8|Clinical Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IV Gastric Cancer AJCC v8|Clinical Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVA Gastric Cancer AJCC v8|Clinical Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Clinical Stage IVB Gastric Cancer AJCC v8|Clinical Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|HER2-Positive Breast Carcinoma|Malignant Hepatobiliary Neoplasm|Metastatic Breast Carcinoma|Metastatic Gastroesophageal Junction Adenocarcinoma|Metastatic Malignant Solid Neoplasm|Pathologic Stage III Gastric Cancer AJCC v8|Pathologic Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIA Gastric Cancer AJCC v8|Pathologic Stage IIIA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIB Gastric Cancer AJCC v8|Pathologic Stage IIIB Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IIIC Gastric Cancer AJCC v8|Pathologic Stage IV Gastric Cancer AJCC v8|Pathologic Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8|Pathologic Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8|Prognostic Stage III Breast Cancer AJCC v8|Prognostic Stage IIIA Breast Cancer AJCC v8|Prognostic Stage IIIB Breast Cancer AJCC v8|Prognostic Stage IIIC Breast Cancer AJCC v8|Prognostic Stage IV Breast Cancer AJCC v8|Stage III Colon Cancer AJCC v8|Stage III Colorectal Cancer AJCC v8|Stage III Major Salivary Gland Cancer AJCC v8|Stage III Uterine Corpus Cancer AJCC v8|Stage IIIA Colon Cancer AJCC v8|Stage IIIA Colorectal Cancer AJCC v8|Stage IIIA Uterine Corpus Cancer AJCC v8|Stage IIIB Colon Cancer AJCC v8|Stage IIIB Colorectal Cancer AJCC v8|Stage IIIB Uterine Corpus Cancer AJCC v8|Stage IIIC Colon Cancer AJCC v8|Stage IIIC Colorectal Cancer AJCC v8|Stage IIIC Uterine Corpus Cancer AJCC v8|Stage IIIC1 Uterine Corpus Cancer AJCC v8|Stage IIIC2 Uterine Corpus Cancer AJCC v8|Stage IV Colon Cancer AJCC v8|Stage IV Colorectal Cancer AJCC v8|Stage IV Major Salivary Gland Cancer AJCC v8|Stage IV Uterine Corpus Cancer AJCC v8|Stage IVA Colon Cancer AJCC v8|Stage IVA Colorectal Cancer AJCC v8|Stage IVA Major Salivary Gland Cancer AJCC v8|Stage IVA Uterine Corpus Cancer AJCC v8|Stage IVB Colon Cancer AJCC v8|Stage IVB Colorectal Cancer AJCC v8|Stage IVB Major Salivary Gland Cancer AJCC v8|Stage IVB Uterine Corpus Cancer AJCC v8|Stage IVC Colon Cancer AJCC v8|Stage IVC Colorectal Cancer AJCC v8|Stage IVC Major Salivary Gland Cancer AJCC v8|Unresectable Colorectal Carcinoma|Unresectable Gastroesophageal Junction Adenocarcinoma|Unresectable Malignant Solid Neoplasm |
National Cancer Institute (NCI) |
August 9 2021 | Phase 1 |
|
化学信息&溶解度
| 分子量 | 412.51 | 分子式 | C20H24N6O2S |
| CAS号 | 1352226-88-0 | SDF | Download Ceralasertib (AZD6738) SDF |
| Smiles | CC1COCCN1C2=NC(=NC(=C2)C3(CC3)S(=N)(=O)C)C4=C5C=CNC5=NC=C4 | ||
| 储存条件(自收到货起) | |||
|
体外溶解度 |
DMSO : 82 mg/mL ( (198.78 mM) ;DMSO吸湿会降低化合物溶解度,请使用新开封DMSO) Ethanol : 82 mg/mL (198.78 mM) Water : Insoluble |
摩尔浓度计算器 |
|
体内溶解配方 现配现用,请按从左到右的顺序依次添加,澄清后再加入下一溶剂 |
动物体内配方计算器 | |||||
实验计算
动物体内配方计算器(澄清溶液)
第一步:请输入基本实验信息(考虑到实验过程中的损耗,建议多配一只动物的药量)
mg/kg
g
μL
只
第二步:请输入动物体内配方组成(配方适用于不溶于水的药物;不同批次药物配方比例不同,请联系Selleck为您提供正确的澄清溶液配方)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
计算结果:
工作液浓度: mg/ml;
DMSO母液配制方法: mg 药物溶于μL DMSO溶液(母液浓度mg/mL,注:如该浓度超过该批次药物DMSO溶解度,请先联系Selleck);
体内配方配制方法:取μL DMSO母液,加入μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入μL ddH2O,混匀澄清。
体内配方配制方法:取μL DMSO母液,加入μL Corn oil,混匀澄清。
注意:1. 首先保证母液是澄清的;
2.一定要按照顺序依次将溶剂加入,进行下一步操作之前必须保证上一步操作得到的是澄清的溶液,可采用涡旋、超声或水浴加热等物理方法助溶。
技术支持
在订购、运输、储存和使用我们的产品的任何阶段,您遇到的任何问题,均可以通过拨打我们的热线电话400-668-6834,或者技术支持邮箱tech@selleck.cn,直接联系到我们。我们会在24小时内尽快联系您。
如果有其他问题,请给我们留言。
* 必填项